Page 63 - 6641
P. 63
hydroxide is formed. The contents of the test tube with Cu
(OH) 2 are poured into three test tubes and each of them add:
a) in the first 2-3 drops of ethanol b) in the second - 2-3
drops of 1,2-ethanediol (ethylene glycol) c) in the third - 2-
3 drops of 1,2,3-propanetriol (glycerol) Shake the contents
of the test tubes and record the observations.
Typical reaction of phenols (hydroxybenzenes)
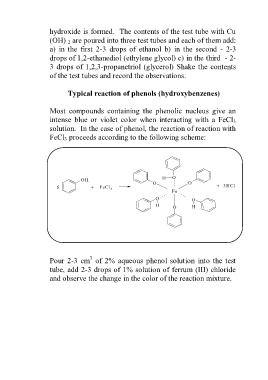
Most compounds containing the phenolic nucleus give an
intense blue or violet color when interacting with a FeCl 3
solution. In the case of phenol, the reaction of reaction with
FeCl 3 proceeds according to the following scheme:
H O
OH O O
6 + FeCl + 3HCl
3 Fe
O O
H O H
3
Pour 2-3 cm of 2% aqueous phenol solution into the test
tube, add 2-3 drops of 1% solution of ferrum (III) chloride
and observe the change in the color of the reaction mixture.