Page 60 - 6641
P. 60
Preparation of chloroethane
3
In a test tube, 2-3 cm pre-cooked ethanol mixture
with concentrated sulfuric acid is poured into the test tube.
Add 1-2 g of sodium chloride, introduce thin glass
capillaries (Fig. 5.1). The test tube is closed with a gas-fired
tube, fixed inclined in the clamp of the tripod and carefully
heated over the burner torch. If after some time to ignite the
gas released through the gas-fired tube, there is a gas
combustion with a characteristic ring of green on the edge of
the gas-fired tube. The presence of a green flame is
characteristic of halogen derivatives, in this case for
chloroetane.
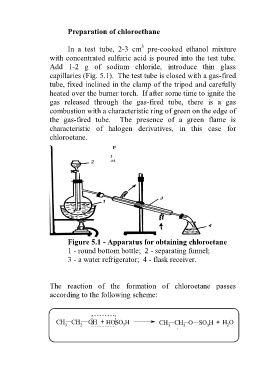
Figure 5.1 - Apparatus for obtaining chloroetane
1 - round bottom bottle; 2 - separating funnel;
3 - a water refrigerator; 4 - flask receiver.
The reaction of the formation of chloroetane passes
according to the following scheme:
CH CH OH + HOSO H CH CH O SO H + H O
3 2 3 3 2 3 2
етанол етилсульфатна кислота