Page 62 - 6641
P. 62
3 CH CH OH + K Cr O + 4H SO
3 2 2 2 7 2 4
O
K SO + Cr (SO ) + 3 CH C + 7H O
2 4 2 4 3 3 H 2
Ethanal ( acetic aldehyde)
3 CH CH CH + K Cr O + 4H SO 4
7
2
2
2
3 3
OH
K SO + Cr (SO ) + 3 CH C CH + 7H O
2 4 2 4 3 3 3 2
O
ethanon
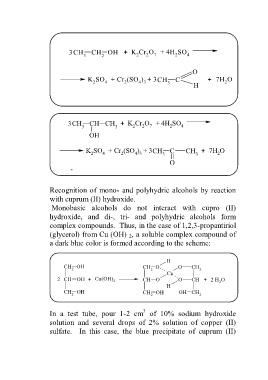
Recognition of mono- and polyhydric alcohols by reaction
with cuprum (II) hydroxide.
Monobasic alcohols do not interact with cupro (II)
hydroxide, and di-, tri- and polyhydric alcohols form
complex compounds. Thus, in the case of 1,2,3-propantiriol
(glycerol) from Cu (OH) 2, a soluble complex compound of
a dark blue color is formed according to the scheme:
H
CH OH CH O O CH 2
2
2
Cu
2 CH OH + Сu(OH) 2 CH O O CH + 2 H O
2
H
CH OH CH OH OH CH
2 2 2
3
In a test tube, pour 1-2 cm of 10% sodium hydroxide
solution and several drops of 2% solution of copper (II)
sulfate. In this case, the blue precipitate of cuprum (II)