Page 23 - 4498
P. 23
1.12. Ttransport Phenomenon Mean Frequency of Molecules
Collisions in Gas. Mean Free Path
In physics, chemistry, biology and engineering, a transport
phenomenon is any of various mechanisms by which particles or
quantities move from one place to another. Due to such movement the
particles may transport mass, heat or momentum. For example if
molecules transport mass we observe diffusion. Heat transfer is transport
energy. The cause of internal friction or viscosity is momentum transfer.
Transport phenomena in gases are studied by the kinetic theory of gases,
primarily by the mean free path of molecules
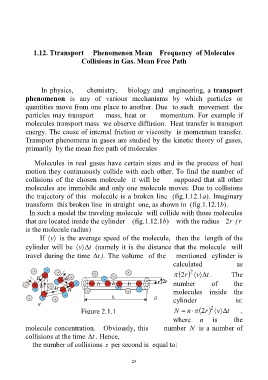
Molecules in real gases have certain sizes and in the process of heat
motion they continuously collide with each other. To find the number of
collisions of the chosen molecule it will be supposed that all other
molecules are immobile and only one molecule moves. Due to collisions
the trajectory of this molecule is a broken line (fig.1.12.1a). Imaginary
transform this broken line in straight one, as shown in (fig.1.12.1b).
In such a model the traveling molecule will collide with those molecules
that are located inside the cylinder (fig.1.12.1b) with the radius r2 (r
is the molecule radius)
If v is the average speed of the molecule, then the length of the
cylinder will be v t (namely it is the distance that the molecule will
travel during the time t ). The volume of the mentioned cylinder is
calculated as
2
vr 2 t . The
number of the
molecules inside the
б cylinder is:
а
Figure 2.1.1 N n vr 2 2 t ,
where n is the
molecule concentration. Obviously, this number N is a number of
collisions at the time t . Hence,
the number of collisions z per second is equal to:
23