Page 18 - 4498
P. 18
this constant is called the gas constant (or universal gas constant ) denoted
by R.
J mol
In SI system R 31,8 . We obtain the following formula for
K
1 mol of ideal gas:
p V R T (1.8.2)
For arbitrary amount of gas there is such formula:
m
p V RT or p V RT (1.8.3)
M
This equation is named Mendeleev- Clapeyron equation, where m – mass
m m
of gas, M- molar mass, - number of moles. Thus, , then
M V
p RT (1.8.4)
M
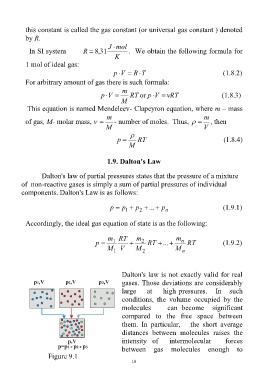
1.9. Dalton's Law
Dalton's law of partial pressures states that the pressure of a mixture
of non-reactive gases is simply a sum of partial pressures of individual
components. Dalton's Law is as follows:
p p p ... p (1.9.1)
2
1
n
Accordingly, the ideal gas equation of state is as the following:
m RT m m
p 1 2 RT ... n RT (1.9.2)
M 1 V M 2 M n
Dalton's law is not exactly valid for real
gases. Those deviations are considerably
large at high pressures. In such
conditions, the volume occupied by the
molecules can become significant
compared to the free space between
them. In particular, the short average
distances between molecules raises the
intensity of intermolecular forces
between gas molecules enough to
Figure 9.1
18