Page 20 - 4498
P. 20
Maxwellian distribution in Fig. 10.1 is equal to unit (S= 1).
To find the most probable speed v pob that corresponds to the maximum
of the function vf (Fig. 10.1) it's necessary to investigate this graph on
extremums, namely to find the derivative and to find the solution of the
following equation:
df v
0 (1.10.4)
dv
Hence the most probable speed is equal to :
2 kT 2 RT
v (1.10.5)
prob
m M
The average speed of a molecule (see fig.10.1) is :
8 kT 8 RT
v (1.10.6)
m M
And the root-mean-square speed is equal to
3 kT 3 RT
v rms (1.10.7)
m M
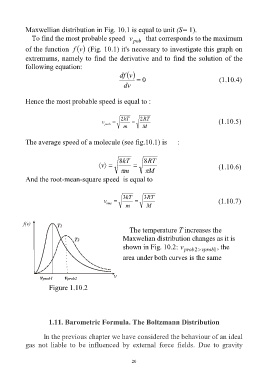
The temperature T increases the
Maxwelian distribution changes as it is
shown in Fig. 10.2: v prob 2 vprob 1 , the
area under both curves is the same
Figure 1.10.2
1.11. Barometric Formula. The Boltzmann Distribution
In the previous chapter we have considered the behaviour of an ideal
gas not liable to be influenced by external force fields. Due to gravity
20