Page 27 - 4498
P. 27
1 1
J v 2 v (1.13.7)
1
6 6
set (1.13.7) equal to (1.13.3) and if we take into account Z 2 l ,
we obtain that diffusion coefficient of ideal gas equal to
1
D v l (1.13.8)
3
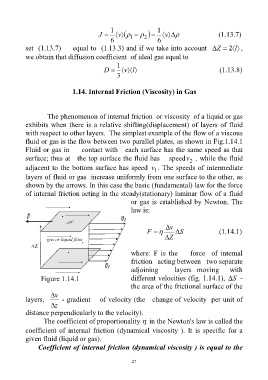
1.14. Internal Friction (Viscosity) in Gas
The phenomenon of internal friction or viscosity of a liquid or gas
exhibits when there is a relative shifting(displacement) of layers of fluid
with respect to other layers. The simplest example of the flow of a viscous
fluid or gas is the flow between two parallel plates, as shown in Fig.1.14.1
Fluid or gas in contact with each surface has the same speed as that
surface; thus at the top surface the fluid has speedv , while the fluid
2
adjacent to the bottom surface has speed v . The speeds of intermediate
1
layers of fluid or gas increase uniformly from one surface to the other, as
shown by the arrows. In this case the basic (fundamental) law for the force
of internal friction acting in the steady(stationary) laminar flow of a fluid
or gas is established by Newton. The
law is:
v
F S (1.14.1)
Z
where: F is the force of internal
friction acting between two separate
adjoining layers moving with
Figure 1.14.1 different velocities (fig. 1.14.1), S -
the area of the frictional surface of the
v
layers, - gradient of velocity (the change of velocity per unit of
z
distance perpendicularly to the velocity).
The coefficient of proportionality in the Newton's law is called the
coefficient of internal friction (dynamical viscosity ). It is specific for a
given fluid (liquid or gas).
Coefficient of internal friction (dynamical viscosity ) is equal to the
27