Page 17 - 4498
P. 17
p p 1 p 2
const or (1.7.5)
T T 1 T 2
Thus, we received Gay-Lussac's law
Gay-Lussac's law, or the pressure law, was found by Joseph Louis
Gay-Lussac in 1809. It states that the pressure exerted on the sides of a
container at a fixed ideal gas volume is proportional to its temperature.
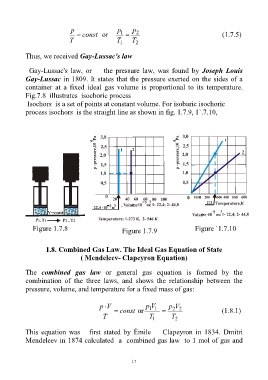
Fig.7.8 illustrates isochoric process
Isochors is a set of points at constant volume. For isobaric isochoric
process isochors is the straight line as shown in fig. 1.7.9, 1`.7.10,
Figure 1.7.8 Figure 1.7.9 Figure `1.7.10
1.8. Combined Gas Law. The Ideal Gas Equation of State
( Mendeleev- Clapeyron Equation)
The combined gas law or general gas equation is formed by the
combination of the three laws, and shows the relationship between the
pressure, volume, and temperature for a fixed mass of gas:
p V p 1 V p V
const or 1 2 2 (1.8.1)
T T 1 T 2
This equation was first stated by Émile Clapeyron in 1834. Dmitri
Mendeleev in 1874 calculated a combined gas law to 1 mol of gas and
17