Page 16 - 4498
P. 16
2. isobaric process
An isobaric process is a thermodynamic process in which the pressure
stays constant: p const.We will rewrite equation (6.3) in the next form
V N k
(1.7.2)
T p
There are three constant values N, k, p in equation (7.2).
Therefore,
V V V
const or 1 2 (1.7.3)
T T 1 T 2
Thus, we received Charles' Law
Charles' Law, or the law of volumes, was found in 1787 by Jacques
Charles. It says that, for an ideal gas at constant pressure, the volume is
directly proportional to its temperature.
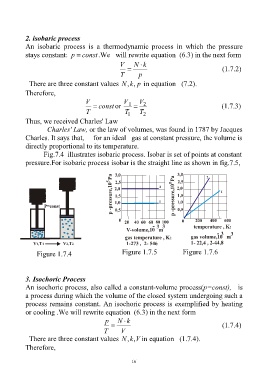
Fig.7.4 illustrates isobaric process. Isobar is set of points at constant
pressure.For isobaric process isobar is the straight line as shown in fig.7.5,
Figure 1.7.4 Figure 1.7.5 Figure 1.7.6
3. Isochoric Process
An isochoric process, also called a constant-volume process(p=const), is
a process during which the volume of the closed system undergoing such a
process remains constant. An isochoric process is exemplified by heating
or cooling .We will rewrite equation (6.3) in the next form
p N k
(1.7.4)
T V
There are three constant values N, k, V in equation (1.7.4).
Therefore,
16