Page 14 - 4498
P. 14
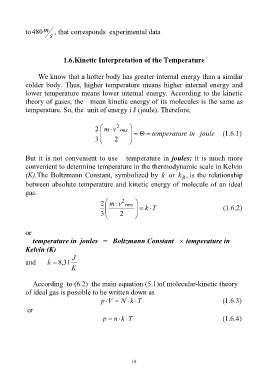
to480 m , that corresponds experimental data
s
1.6.Kinetic Interpretation of the Temperature
We know that a hotter body has greater internal energy than a similar
colder body. Thus, higher temperature means higher internal energy and
lower temperature means lower internal energy. According to the kinetic
theory of gases, the mean kinetic energy of its molecules is the same as
temperature. So, the unit of energy i J (joule). Therefore,
2
2 m v rms
temperatur e in joule (1.6.1)
3 2
But it is not convenient to use temperature in joules; it is much more
convenient to determine temperature in the thermodynamic scale in Kelvin
(K).The Boltzmann Constant, symbolized by k or k , is the relationship
B
between absolute temperature and kinetic energy of molecule of an ideal
gas.
2
2 m v rms
k T (1.6.2)
3 2
or
temperature in joules = Boltzmann Constant temperature in
Kelvin (K)
J
and k , 8 31
K
According to (6.2) the main equation (5.1)of molecular-kinetic theory
of ideal gas is possible to be written down as
p V N k T (1.6.3)
or
p n k T (1.6.4)
14