Page 9 - 4498
P. 9
This definition fixes the magnitude of both the degree Celsius and the
kelvin as precisely 1 part in 273.16 (approximately 0.00366) of the
difference between absolute zero and the triple point of water. Thus, it sets
the magnitude of one degree Celsius and that of one kelvin as exactly the
same. Additionally, it establishes the difference between the two scales'
null points as being precisely 273.15 degrees Celsius (−273.15 °C = 0 K
and 0 °C = 273.15 K).
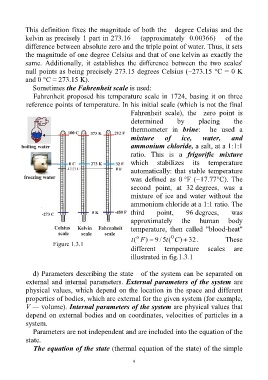
Sometimes the Fahrenheit scale is used:
Fahrenheit proposed his temperature scale in 1724, basing it on three
reference points of temperature. In his initial scale (which is not the final
Fahrenheit scale), the zero point is
determined by placing the
thermometer in brine: he used a
mixture of ice, water, and
ammonium chloride, a salt, at a 1:1:1
ratio. This is a frigorific mixture
which stabilizes its temperature
automatically: that stable temperature
was defined as 0 °F (−17.77°C). The
second point, at 32 degrees, was a
mixture of ice and water without the
ammonium chloride at a 1:1 ratio. The
third point, 96 degrees, was
approximately the human body
temperature, then called "blood-heat"
o 0
t ( F ) 5 / 9 t ( C ) 32. These
Figure 1.3.1
different temperature scales are
illustrated in fig.1.3.1
d) Parameters describing the state of the system can be separated on
external and internal parameters. External parameters of the system are
physical values, which depend on the location in the space and different
properties of bodies, which are external for the given system (for example,
V — volume). Internal parameters of the system are physical values that
depend on external bodies and on coordinates, velocities of particles in a
system.
Parameters are not independent and are included into the equation of the
state.
The equation of the state (thermal equation of the state) of the simple
9