Page 20 - 6641
P. 20
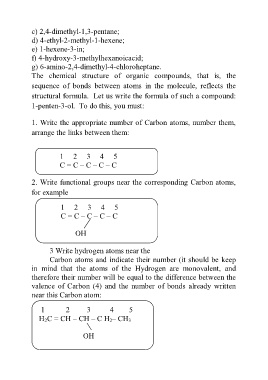
c) 2,4-dimethyl-1,3-pentane;
d) 4-ethyl-2-methyl-1-hexene;
e) 1-hexene-3-in;
f) 4-hydroxy-3-methylhexanoicacid;
g) 6-amino-2,4-dimethyl-4-chloroheptane.
The chemical structure of organic compounds, that is, the
sequence of bonds between atoms in the molecule, reflects the
structural formula. Let us write the formula of such a compound:
1-penten-3-ol. To do this, you must:
1. Write the appropriate number of Carbon atoms, number them,
arrange the links between them:
1 2 3 4 5
С = С – С – С – С
2. Write functional groups near the corresponding Carbon atoms,
for example
1 2 3 4 5
С = С – С – С – С
OH
3 Write hydrogen atoms near the
Carbon atoms and indicate their number (it should be keep
in mind that the atoms of the Hydrogen are monovalent, and
therefore their number will be equal to the difference between the
valence of Carbon (4) and the number of bonds already written
near this Carbon atom:
1 2 3 4 5
H 2С = СH – СH – С H 2– СH 3
OH