Page 22 - 6641
P. 22
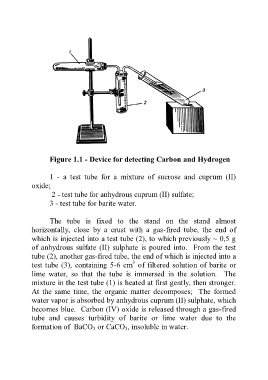
Figure 1.1 - Device for detecting Carbon and Hydrogen
1 - a test tube for a mixture of sucrose and cuprum (II)
oxide;
2 - test tube for anhydrous cuprum (II) sulfate;
3 - test tube for barite water.
The tube is fixed to the stand on the stand almost
horizontally, close by a crust with a gas-fired tube, the end of
which is injected into a test tube (2), to which previously ~ 0,5 g
of anhydrous sulfate (II) sulphate is poured into. From the test
tube (2), another gas-fired tube, the end of which is injected into a
3
test tube (3), containing 5-6 cm of filtered solution of barite or
lime water, so that the tube is immersed in the solution. The
mixture in the test tube (1) is heated at first gently, then stronger.
At the same time, the organic matter decomposes; The formed
water vapor is absorbed by anhydrous cuprum (II) sulphate, which
becomes blue. Carbon (IV) oxide is released through a gas-fired
tube and causes turbidity of barite or lime water due to the
formation of BaСО 3 or СаСО 3, insoluble in water.