Page 24 - 6641
P. 24
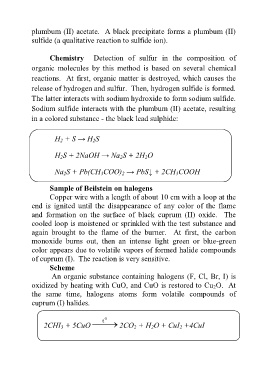
plumbum (II) acetate. A black precipitate forms a plumbum (II)
sulfide (a qualitative reaction to sulfide ion).
Chemistry Detection of sulfur in the composition of
organic molecules by this method is based on several chemical
reactions. At first, organic matter is destroyed, which causes the
release of hydrogen and sulfur. Then, hydrogen sulfide is formed.
The latter interacts with sodium hydroxide to form sodium sulfide.
Sodium sulfide interacts with the plumbum (II) acetate, resulting
in a colored substance - the black lead sulphide:
H 2 + S → H 2S
H 2S + 2NaOH → Na 2S + 2H 2O
Na 2S + Pb(CH 3COO) 2 → PbS↓ + 2CH 3COOH
Sample of Beilstein on halogens
Copper wire with a length of about 10 cm with a loop at the
end is ignited until the disappearance of any color of the flame
and formation on the surface of black cuprum (II) oxide. The
cooled loop is moistened or sprinkled with the test substance and
again brought to the flame of the burner. At first, the carbon
monoxide burns out, then an intense light green or blue-green
color appears due to volatile vapors of formed halide compounds
of cuprum (I). The reaction is very sensitive.
Scheme
An organic substance containing halogens (F, Cl, Br, I) is
oxidized by heating with CuO, and CuO is restored to Cu 2O. At
the same time, halogens atoms form volatile compounds of
cuprum (I) halides.
2CHI 3 + 5CuO t 0 2CO 2 + H 2O + CuІ 2 +4CuІ