Page 78 - 4498
P. 78
2
until the weight of the liquid supported, mg gV gh r , is equal to
this force. Hence, for the equilibrium
2
2 r cos gh r (3.7.1)
or
2 cos
h (3.7.2)
gr
For water in a clean glass the angle is zero, and cos cos0 becomes a
unity. Whenever a liquid wets a tube thoroughly, is zero. When cohesive
forces exceed the adhesive ones, is greater than 90°, the liquid is depressed
in the capillary, and h is negative. For water on paraffin is 107°. Water does
not wet paraffin. For mercury on glass the angle of contact is approximately
140°.
CHAPTER 4
Solid Body
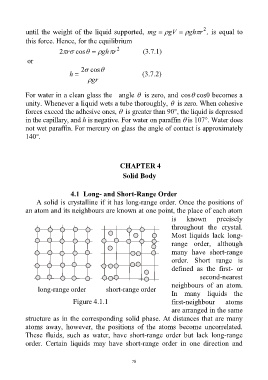
4.1 Long- and Short-Range Order
A solid is crystalline if it has long-range order. Once the positions of
an atom and its neighbours are known at one point, the place of each atom
is known precisely
throughout the crystal.
Most liquids lack long-
range order, although
many have short-range
order. Short range is
defined as the first- or
second-nearest
neighbours of an atom.
long-range order short-range order
In many liquids the
Figure 4.1.1 first-neighbour atoms
are arranged in the same
structure as in the corresponding solid phase. At distances that are many
atoms away, however, the positions of the atoms become uncorrelated.
These fluids, such as water, have short-range order but lack long-range
order. Certain liquids may have short-range order in one direction and
78