Page 77 - 4498
P. 77
A 1 mm bubble has negligible extra pressure. Yet when the diameter is
~3 µm, the bubble has an extra atmosphere inside and not outside. When
the bubble is only several hundred nanometers, the pressure inside can be
several atmospheres. One should bear in mind that the surface tension in
the numerator can be much smaller in the presence of surfactants or
contaminants. The same calculation can be done for small oil droplets in
water, where even in the presence of surfactants and a fairly low interfacial
tension 5 10 mN , the pressure inside 100 nm diameter droplets can
m
reach several atmospheres.
3.7 Capillary Action
Capillary action, or capillarity, is the tendency of liquids to rise or to be
depressed in tubes of small diameter. Capillarity is due to the adhesion of
the liquid to the sides of the tube, and to the
surface tension of the liquid.
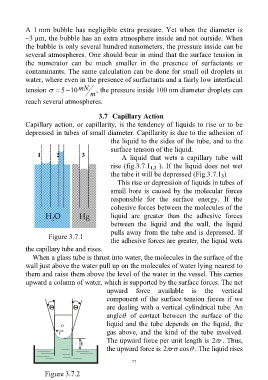
1 2 3
A liquid that wets a capillary tube will
rise (fig.3.7.1 ). If the liquid does not wet
1,2
the tube it will be depressed (Fig.3.7.1 ).
3
This rise or depression of liquids in tubes of
small bore is caused by the molecular forces
responsible for the surface energy. If the
cohesive forces between the molecules of the
liquid are greater than the adhesive forces
between the liquid and the wall, the liquid
pulls away from the tube and is depressed. If
Figure 3.7.1
the adhesive forces are greater, the liquid wets
the capillary tube and rises.
When a glass tube is thrust into water, the molecules in the surface of the
wall just above the water pull up on the molecules of water lying nearest to
them and raise them above the level of the water in the vessel. This carries
upward a column of water, which is supported by the surface forces. The net
upward force available is the vertical
component of the surface tension forces if we
are dealing with a vertical cylindrical tube. An
angle of contact between the surface of the
liquid and the tube depends on the liquid, the
gas above, and the kind of the tube involved.
The upward force per unit length is 2 r. Thus,
the upward force is 2 r cos . The liquid rises
77
Figure 3.7.2