Page 74 - 4498
P. 74
surfaces). Thus, multiplying both the numerator and the denominator of
1 F
by dx, we get
2 L
1 F Fdx dA
(3.5.2)
2 L 2 Ldx dS
This work is, by the usual arguments, interpreted as being stored as
potential energy. Consequently surface tension can be also measured in SI
system as joules per square meter. Since mechanical systems try to find a
state of minimum potential energy, a free droplet of liquid naturally
assumes a spherical shape, which has the minimum surface area for a
given volume.
3.6. Wetting
Wetting is a phenomenon arising upon contact of a liquid with the
surface of a solid or other liquid. In particular, wetting is seen in the
spreading of a liquid over a solid surface in contact with a gas (vapor) or
other liquid, in the impregnation of porous bodies and powders, and in the
change in shape of the surface of a liquid at the surface of a solid. Thus,
wetting results in the formation of a spherical meniscus in a capillary tube
and determines the shape of a droplet on a solid surface or the shape of a
gas bubble on the surface of an object immersed in a liquid. Wetting is
often regarded to as a result of intermolecular (van der Waals’)
interactions in the zone of contact of three phases (bodies, media).
However, in many cases, for example, in the contact of liquid metals with
solid metals, oxides, diamond, and graphite, wetting is caused more by the
formation of chemical bonds, solid and liquid solutions, and diffusion
processes in the surface layer of the wetted body than by intermolecular
interactions. The heat effect accompanying the contact of a liquid with the
surface being wetted is called the heat of wetting.
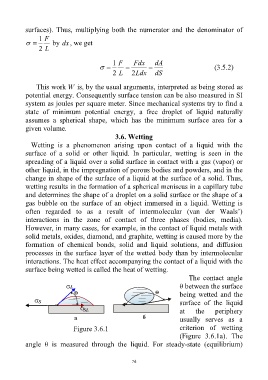
The contact angle
θ between the surface
being wetted and the
surface of the liquid
at the periphery
usually serves as a
Figure 3.6.1 criterion of wetting
(Figure 3.6.1a). The
angle θ is measured through the liquid. For steady-state (equilibrium)
74