Page 71 - 4498
P. 71
lowers the temperature of the remaining part of the compressed gas 1–M.
This remaining part is then throttled and liquefied. In theory, expansion in
an expander should be carried out at constant entropy (3–6). Because of
losses, however, the expansion proceeds along line 3–7. To increase the
thermodynamic efficiency of the process of gas liquefaction, several
expanders operating at different temperature levels are sometimes used.
Cycles with heat pumps are usually used (in addition to expansion
and throttling cycles) in liquefying gases by means of gas refrigerators,
which make it possible to obtain temperatures as low as 12°K. These
temperatures are low enough to liquefy all gases except helium (see
Table 3.4.1). An additional throttling stage is attached to the refrigerator to
liquefy helium.
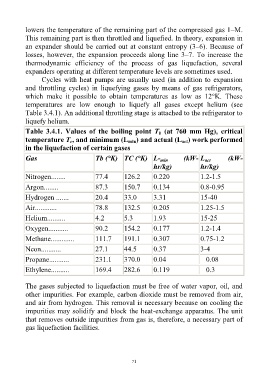
Table 3.4.1. Values of the boiling point T (at 760 mm Hg), critical
b
temperature T , and minimum (L min ) and actual (L ) work performed
c
act
in the liquefaction of certain gases
Gas Tb (°K) TC (°K) L- min (kW- L (kW-
act
hr/kg) hr/kg)
Nitrogen........ 77.4 126.2 0.220 1.2-1.5
Argon........ 87.3 150.7 0.134 0.8-0.95
Hydrogen ....... 20.4 33.0 3.31 15-40
Air............ 78.8 132.5 0.205 1.25-1.5
Helium.......... 4.2 5.3 1.93 15-25
Oxygen........... 90.2 154.2 0.177 1.2-1.4
Methane............. 111.7 191.1 0.307 0.75-1.2
Neon........... 27.1 44.5 0.37 3-4
Propane........... 231.1 370.0 0.04 0.08
Ethylene.......... 169.4 282.6 0.119 0.3
The gases subjected to liquefaction must be free of water vapor, oil, and
other impurities. For example, carbon dioxide must be removed from air,
and air from hydrogen. This removal is necessary because on cooling the
impurities may solidify and block the heat-exchange apparatus. The unit
that removes outside impurities from gas is, therefore, a necessary part of
gas liquefaction facilities.
71