Page 66 - 4498
P. 66
interaction of real gas to cause change of kinetic energy of gas molecules
and, as a result, change of temperature of this real gas.
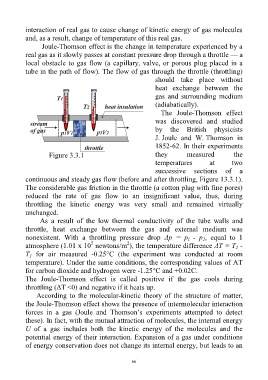
Joule-Thomson effect is the change in temperature experienced by a
real gas as it slowly passes at constant pressure drop through a throttle — a
local obstacle to gas flow (a capillary, valve, or porous plug placed in a
tube in the path of flow). The flow of gas through the throttle (throttling)
should take place without
heat exchange between the
gas and surrounding medium
(adiabatically).
The Joule-Thomson effect
was discovered and studied
by the British physicists
J. Joule and W. Thomson in
1852-62. In their experiments
Figure 3.3.1 they measured the
temperatures at two
successive sections of a
continuous and steady gas flow (before and after throttling, Figure 13.3.1).
The considerable gas friction in the throttle (a cotton plug with fine pores)
reduced the rate of gas flow to an insignificant value, thus, during
throttling the kinetic energy was very small and remained virtually
unchanged.
As a result of the low thermal conductivity of the tube walls and
throttle, heat exchange between the gas and external medium was
nonexistent. With a throttling pressure drop Δp = p - p , equal to 1
2
l
2
5
atmosphere (1.01 x 10 newtons/m ), the temperature difference ΔT = T -
2
T for air measured -0.25°C (the experiment was conducted at room
1
temperature). Under the same conditions, the corresponding values of AT
for carbon dioxide and hydrogen were -1.25°C and +0.02C.
The Joule-Thomson effect is called positive if the gas cools during
throttling (ΔT <0) and negative if it heats up.
According to the molecular-kinetic theory of the structure of matter,
the Joule-Thomson effect shows the presence of intermolecular interaction
forces in a gas (Joule and Thomson’s experiments attempted to detect
these). In fact, with the mutual attraction of molecules, the internal energy
U of a gas includes both the kinetic energy of the molecules and the
potential energy of their interaction. Expansion of a gas under conditions
of energy conservation does not change its internal energy, but leads to an
66