Page 64 - 4498
P. 64
a a 8
p ; V 3 b ; T (3.2.10)
C 2 C C
27 b 27 b R
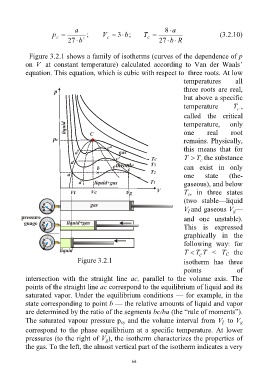
Figure 3.2.1 shows a family of isotherms (curves of the dependence of p
on V at constant temperature) calculated according to Van der Waals’
equation. This equation, which is cubic with respect to three roots. At low
temperatures all
three roots are real,
but above a specific
temperature T ,
C
called the critical
temperature, only
one real root
remains. Physically,
this means that for
T T the substance
C
can exist in only
one state (the-
gaseous), and below
T , in three states
c
(two stable—liquid
V and gaseous V —
l
g
and one unstable).
This is expressed
graphically in the
following way: for
T T T < T the
c
C
Figure 3.2.1 isotherm has three
points of
intersection with the straight line ac, parallel to the volume axis. The
points of the straight line ac correspond to the equilibrium of liquid and its
saturated vapor. Under the equilibrium conditions — for example, in the
state corresponding to point b — the relative amounts of liquid and vapor
are determined by the ratio of the segments bc/ba (the “rule of moments”).
The saturated vapour pressure p and the volume interval from V to V
sy
l
g
correspond to the phase equilibrium at a specific temperature. At lower
pressures (to the right of V ), the isotherm characterizes the properties of
g
the gas. To the left, the almost vertical part of the isotherm indicates a very
64