Page 63 - 4498
P. 63
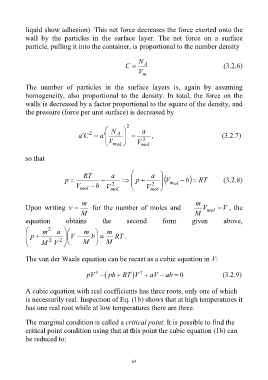
liquid show adhesion). This net force decreases the force exerted onto the
wall by the particles in the surface layer. The net force on a surface
particle, pulling it into the container, is proportional to the number density
N
C A (3.2.6)
V m
The number of particles in the surface layers is, again by assuming
homogeneity, also proportional to the density. In total, the force on the
walls is decreased by a factor proportional to the square of the density, and
the pressure (force per unit surface) is decreased by
2
N a
Ca 2 a A , (3.2.7)
V 2
m ol V mol
so that
RT a a
p p V m ol b RT (3.2.8)
V b V 2 V 2
mol mol mol
m m
Upon writing for the number of moles and V mol V , the
M M
equation obtains the second form given above,
m 2 a m m
p V b RT .
2 2 M M
M V
The van der Waals equation can be recast as a cubic equation in V:
3
2
pV pb RT V aV ab 0 (3.2.9)
A cubic equation with real coefficients has three roots, only one of which
is necessarily real. Inspection of Eq. (1b) shows that at high temperatures it
has one real root while at low temperatures there are three.
The marginal condition is called a critical point. It is possible to find the
critical point condition using that at this point the cubic equation (1b) can
be reduced to:
63