Page 71 - 6641
P. 71
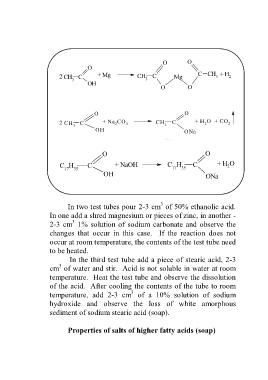
O O
O
2 CH C + Mg CH C Mg C CH + H 2
3
3 3
OH
O O
O O
2 CH 3 C + Na CO 3 CH 3 C + H O + CO 2
2
2
OH ONa
етанова кислота натрій етаноат
O O
C H C + NaOH C H C + H O
2
17 35 17 35
OH ONa
3
In two test tubes pour 2-3 cm of 50% ethanolic acid.
In one add a shred magnesium or pieces of zinc, in another -
3
2-3 cm 1% solution of sodium carbonate and observe the
changes that occur in this case. If the reaction does not
occur at room temperature, the contents of the test tube need
to be heated.
In the third test tube add a piece of stearic acid, 2-3
3
cm of water and stir. Acid is not soluble in water at room
temperature. Heat the test tube and observe the dissolution
of the acid. After cooling the contents of the tube to room
3
temperature, add 2-3 cm of a 10% solution of sodium
hydroxide and observe the loss of white amorphous
sediment of sodium stearic acid (soap).
Properties of salts of higher fatty acids (soap)