Page 70 - 6641
P. 70
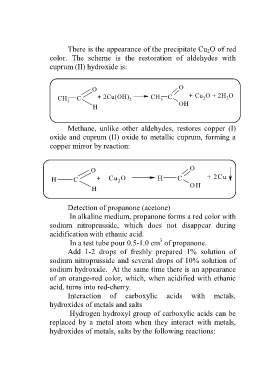
There is the appearance of the precipitate Cu 2O of red
color. The scheme is the restoration of aldehydes with
cuprum (II) hydroxide is:
O O
CH C + 2Cu(OH) 2 CH 3 C + Cu O + 2H O
2
2
3 OH
H осад
карбонова
альдегід
Methane, unlike other aldehydes, restores copper (I)
oxide and cuprum (II) oxide to metallic cuprum, forming a
copper mirror by reaction:
O O
H C + Cu O H C + 2Cu
2
H метанова O H
Detection of propanone (acetone)
In alkaline medium, propanone forms a red color with
sodium nitroprusside, which does not disappear during
acidification with ethanic acid.
3
In a test tube pour 0.5-1.0 cm of propanone.
Add 1-2 drops of freshly prepared 1% solution of
sodium nitroprusside and several drops of 10% solution of
sodium hydroxide. At the same time there is an appearance
of an orange-red color, which, when acidified with ethanic
acid, turns into red-cherry.
Interaction of carboxylic acids with metals,
hydroxides of metals and salts
Hydrogen hydroxyl group of carboxylic acids can be
replaced by a metal atom when they interact with metals,
hydroxides of metals, salts by the following reactions: