Page 39 - 6273
P. 39
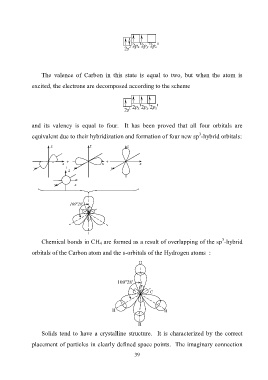
1
1
0
2 2p х 2p y 2p z
2s
The valence of Carbon in this state is equal to two, but when the atom is
excited, the electrons are decomposed according to the scheme
1
1
1
1 2p х 2p y 2p z
2s
and its valency is equal to four. It has been proved that all four orbitals are
3
equivalent due to their hybridization and formation of four new sp -hybrid orbitals:
z z z
+ +
x x x
y + y y
z
y x
о
109 28'
3
Chemical bonds in CH 4 are formed as a result of overlapping of the sp -hybrid
orbitals of the Carbon atom and the s-orbitals of the Hydrogen atoms :
H
· ·
о
109 28'
C
· ·
· ·
H H
· ·
H
Solids tend to have a crystalline structure. It is characterized by the correct
placement of particles in clearly defined space points. The imaginary connection
39