Page 38 - 6273
P. 38
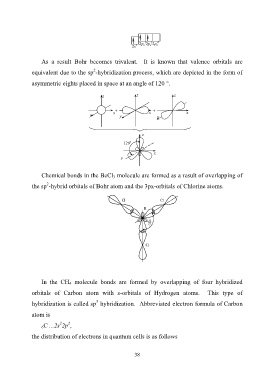
1
1
0
2p х 2p y 2p z
2s 1
As a result Bohr becomes trivalent. It is known that valence orbitals are
2
equivalent due to the sp -hybridization process, which are depicted in the form of
asymmetric eights placed in space at an angle of 120 °.
z z z
+ +
x x x
y y y
z
120 о
x
y
Chemical bonds in the BеCl 3 molecule are formed as a result of overlapping of
2
the sp -hybrid orbitals of Bohr atom and the 3px-orbitals of Chlorine atoms.
In the CH 4 molecule bonds are formed by overlapping of four hybridized
orbitals of Carbon atom with s-orbitals of Hydrogen atoms. This type of
3
hybridization is called sp hybridization. Abbreviated electron formula of Carbon
atom is
2
2
6C ...2s 2p ,
the distribution of electrons in quantum cells is as follows
38