Page 37 - 6273
P. 37
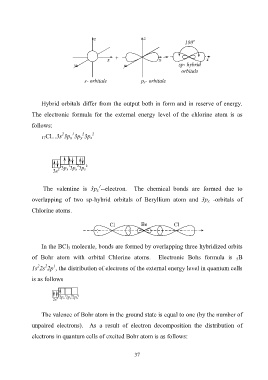
z z о
180
x + x x
y y sp- hybrid
orbitals
s- orbitale p x- orbitale
Hybrid orbitals differ from the output both in form and in reserve of energy.
The electronic formula for the external energy level of the chlorine atom is as
follows:
2 1 2 2
17Cl...3s 3p x 3p y 3p z
1
2
2
2 3p х 3p y 3p z
3s
1
The valentine is 3p x --electron. The chemical bonds are formed due to
overlapping of two sр-hybrid orbitals of Beryllium atom and 3р х -orbitals of
Chlorine atoms.
Cl Be Cl
· ·
· ·
In the BСl 3 molecule, bonds are formed by overlapping three hybridized orbits
of Bohr atom with orbital Chlorine atoms. Electronic Bohs formula is 5B
1
2
2
1s 2s 2p , the distribution of electrons of the external energy level in quantum cells
is as follows
0
0
1
2 2p х 2p y 2p z
2s
The valence of Bohr atom in the ground state is equal to one (by the number of
unpaired electrons). As a result of electron decomposition the distribution of
electrons in quantum cells of excited Bohr atom is as follows:
37