Page 11 - 4911
P. 11
1400
К 2' 3
1200
1000
4
800
6 5
600
2
Т
400
4'
200
0
0,5 1,0 1,5 2,0 2,5 3,0 кДж 3,5
S
кг К
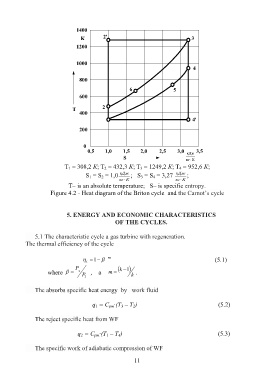
T 1 = 308,2 К; Т 2 = 432,3 К; Т 3 = 1249,2 К; Т 4 = 952,6 К;
S 1 = S 2 = 1,0 кДж ; S 3 = S 4 = 3,27 кДж ;
кг К кг К
T– is an absolute temperature; S– is specific entropy.
Figure 4.2 - Heat diagram of the Briton cycle and the Carnot’s cycle
5. ENERGY AND ECONOMIC CHARACTERISTICS
OF THE CYCLES.
5.1 The characteristic cycle a gas turbine with regeneration.
The thermal efficiency of the cycle
t 1 m (5.1)
P k 1
where 2 P 1 , а m k .
The absorbs specific heat energy by work fluid
q 1 = C pm·(T 3 – T 2) (5.2)
The reject specific heat from WF
q 2 = C pm·(T 1 – T 4) (5.3)
The specific work of adiabatic compression of WF
11