Page 10 - 4911
P. 10
4.4 To determine the coordinates of the isobar’s intermediate points required to break
range of temperature change of WF not less than ten intervals and for each of them to
determine ΔS. For the calculation changing of the specific entropy it is necessary use
the ratio
T i 1
S i C pm ln T (4.1)
i
where S - is the entropy change of WF in the interval [Ti,Ti+1], kJ/(K•kg);
i
T i - is the temperature of the WF at the beginning of the interval, K;
T i+1 - is the temperature of the WF at the end of the interval, K.
As in the study of thermodynamic processes it is important to know not the absolute
value of entropy, but only a change it. Therefore, start of its counting, you can select
arbitrary, for example, 1 kJ/(kg•K) at a minimum temperature cycle.
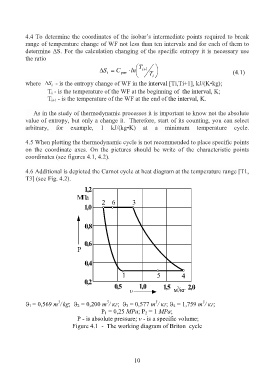
4.5 When plotting the thermodynamic cycle is not recommended to place specific points
on the coordinate axes. On the pictures should be write of the characteristic points
coordinates (see figures 4.1, 4.2).
4.6 Additional is depicted the Carnot cycle at heat diagram at the temperature range [T1,
T3] (see Fig. 4.2).
1,2
МПа
2 6 3
1,0
0,8
0,6
P
0,4
1 5 4
0,2
0,5 1,0 1,5 3 2,0
м /кг
3
3
3
3
1 = 0,569 m / kg; 2 = 0,200 m / кг; 3 = 0,577 m / кг; 4 = 1,759 m / кг;
P 1 = 0,25 МPа; P 2 = 1 МPа;
Р - is absolute pressure; v - is a specific volume;
Figure 4.1 - The working diagram of Briton cycle
10