Page 89 - 4498
P. 89
discontinuously, as a result of some external condition, such as
temperature, pressure, and others. For example, a liquid may become gas
upon heating to the boiling point, resulting in an abrupt change in volume.
The measurement of the external conditions at which the transformation
occurs is termed the phase transition. Phase transitions are common in
nature and used today in many technologies.
In the modern classification scheme, phase transitions are divided into two
broad categories.
First-order phase transitions are those that involve a latent heat.
During such a transition, a system
either absorbs or releases a fixed
(and typically large) amount of
energy. During this process, the
temperature of the system will stay
constant as heat is added: the system
is in a "mixed-phase regime" in
which some parts of the system have
completed the transition and others
have not. Familiar examples are the
melting of ice or the boiling of water
(water does not instantly turn into
vapor, but forms a turbulent mixture
of liquid water and vapor bubbles).
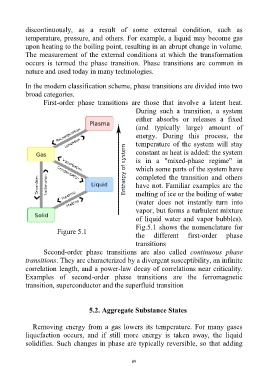
Fig.5.1 shows the nomenclature for
Figure 5.1
the different first-order phase
transitions
Second-order phase transitions are also called continuous phase
transitions. They are characterized by a divergent susceptibility, an infinite
correlation length, and a power-law decay of correlations near criticality.
Examples of second-order phase transitions are the ferromagnetic
transition, superconductor and the superfluid transition
5.2. Aggregate Substance States
Removing energy from a gas lowers its temperature. For many gases
liquefaction occurs, and if still more energy is taken away, the liquid
solidifies. Such changes in phase are typically reversible, so that adding
89