Page 93 - 4498
P. 93
and liquid can exist together without any of them gaining the mass at the
expense of the others. At this temperature and pressure, the solid, liquid,
and vapor are in equilibrium.
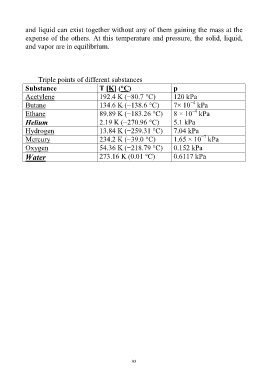
Triple points of different substances
Substance T [K] (°C) p
Acetylene 192.4 K (−80.7 °C) 120 kPa
−4
Butane 134.6 K (−138.6 °C) 7× 10 kPa
−4
Ethane 89.89 K (−183.26 °C) 8 × 10 kPa
Helium 2.19 K (−270.96 °C) 5.1 kPa
Hydrogen 13.84 K (−259.31 °C) 7.04 kPa
−7
Mercury 234.2 K (−39.0 °C) 1.65 × 10 kPa
Oxygen 54.36 K (−218.79 °C) 0.152 kPa
Water 273.16 K (0.01 °C) 0.6117 kPa
93