Page 92 - 4498
P. 92
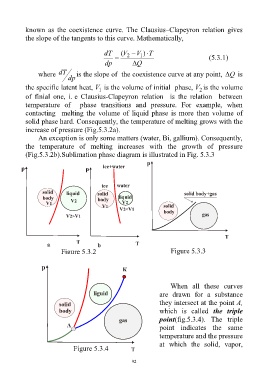
known as the coexistence curve. The Clausius–Clapeyron relation gives
the slope of the tangents to this curve. Mathematically,
dT ( V V 1 T )
2
(5.3.1)
dp Q
where dT is the slope of the coexistence curve at any point, Q is
dp
the specific latent heat, V is the volume of initial phase, V is the volume
1
2
of finial one, i. e Clausius-Clapeyron relation is the relation between
temperature of phase transitions and pressure. For example, when
contacting melting the volume of liquid phase is more then volume of
solid phase hard. Consequently, the temperature of melting grows with the
increase of pressure (Fig.5.3.2a).
An exception is only some matters (water, Bi, gallium). Consequently,
the temperature of melting increases with the growth of pressure
(Fig.5.3.2b).Sublimation phase diagram is illustrated in Fig. 5.3.3
Figure 5.3.2 Figure 5.3.3
When all these curves
are drawn for a substance
they intersect at the point A,
which is called the triple
point(fig.5.3.4). The triple
point indicates the same
temperature and the pressure
at which the solid, vapor,
Figure 5.3.4
92