Page 91 - 4498
P. 91
point of a liquid is the temperature at which the vapor pressure is the same
as the applied pressure, it follows at once that when the applied pressure is
changed, the boiling point also changes. The heat energy of vaporization is
equal to
Q r , m (5.2.2)
where m is a mass liquid, L is the specific heat of vaporization .
Under certain conditions of temperature and pressure a liquid may be in
equilibrium with its vapor.
Similarly, a solid may be in equilibrium with its vapor along the
sublimation curve OA. Sublimation is the transition of a substance
directly from the solid to the gas phase without passing through the
intermediate liquid phase. For some substances, such as carbon and
arsenic, sublimation is much easier than evaporation from the melt
The reverse process of sublimation is desublimation, or deposition.
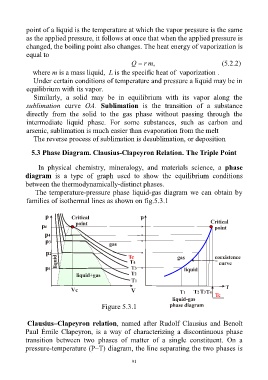
5.3 Phase Diagram. Clausius-Clapeyron Relation. The Triple Point
In physical chemistry, mineralogy, and materials science, a phase
diagram is a type of graph used to show the equilibrium conditions
between the thermodynamically-distinct phases.
The temperature-pressure phase liquid-gas diagram we can obtain by
families of isothermal lines as shown on fig.5.3.1
Figure 5.3.1
Clausius–Clapeyron relation, named after Rudolf Clausius and Benoît
Paul Émile Clapeyron, is a way of characterizing a discontinuous phase
transition between two phases of matter of a single constituent. On a
pressure-temperature (P–T) diagram, the line separating the two phases is
91