Page 84 - 4498
P. 84
Impurities occur because materials are never 100% pure. In the case of an
impurity, the atom is often incorporated at a regular atomic site in the
crystal structure. This is neither a vacant site nor is the atom on an
interstitial site and it is called a substitutional defect (fig.4.4.1c). The
atom is not supposed to be anywhere in the crystal, and is, thus, an
impurity. In some cases where the radius of the substitutional atom (ion) is
substantially smaller than that of the atom (ion) it is replacing, its
equilibrium position can be shifted away from the lattice site. These types
of substitutional defects are often referred to as off-center ions. There are
two different types of substitutional defects: isovalent substitution and
aliovalent substitution. Isovalent substitution is where the ion that is
substituting the original ion is of the same oxidation state as the ion it is
replacing. Aliovalent substitution is where the ion that is substituting the
original ion is of a different oxidation state as the ion it is replacing.
Aliovalent substitutions change the overall charge within the ionic
compound, but the ionic compound must be neutral. Therefore, a charge
compensation mechanism is required. Hence, either of the metals is
partially or fully oxidized or reduced, or ion vacancies are created.
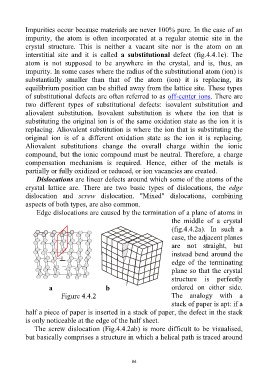
Dislocations are linear defects around which some of the atoms of the
crystal lattice are. There are two basic types of dislocations, the edge
dislocation and screw dislocation. "Mixed" dislocations, combining
aspects of both types, are also common.
Edge dislocations are caused by the termination of a plane of atoms in
the middle of a crystal
(fig.4.4.2a). In such a
case, the adjacent planes
are not straight, but
instead bend around the
edge of the terminating
plane so that the crystal
structure is perfectly
a b ordered on either side.
Figure 4.4.2 The analogy with a
stack of paper is apt: if a
half a piece of paper is inserted in a stack of paper, the defect in the stack
is only noticeable at the edge of the half sheet.
The screw dislocation (Fig.4.4.2ab) is more difficult to be visualised,
but basically comprises a structure in which a helical path is traced around
84