Page 46 - 4498
P. 46
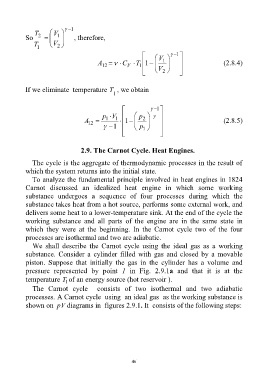
1
T 2 V 1
So , therefore,
T V 2
1
V 1
A 12 C V T 1 1 1 (2.8.4)
V
2
If we eliminate temperature T , we obtain
1
1
p V p
A 12 1 1 1 2 (2.8.5)
1 p 1
2.9. The Carnot Cycle. Heat Engines.
The cycle is the aggregate of thermodynamic processes in the result of
which the system returns into the initial state.
To analyze the fundamental principle involved in heat engines in 1824
Carnot discussed an idealized heat engine in which some working
substance undergoes a sequence of four processes during which the
substance takes heat from a hot source, performs some external work, and
delivers some heat to a lower-temperature sink. At the end of the cycle the
working substance and all parts of the engine are in the same state in
which they were at the beginning. In the Carnot cycle two of the four
processes are isothermal and two are adiabatic.
We shall describe the Carnot cycle using the ideal gas as a working
substance. Consider a cylinder filled with gas and closed by a movable
piston. Suppose that initially the gas in the cylinder has a volume and
pressure represented by point 1 in Fig. 2.9.1a and that it is at the
temperature T of an energy source (hot reservoir ).
1
The Carnot cycle consists of two isothermal and two adiabatic
processes. A Carnot cycle using an ideal gas as the working substance is
shown on pV diagrams in figures 2.9.1. It consists of the following steps:
46