Page 49 - 4498
P. 49
between the same two temperatures. Suppose, steam engine or car engine
are working according to the Carnot cycle, then:
1) Steam engine. 373 K 273K Boiling and freezing water.
, 0 27
373K
2700K
2.Car engine. 300K , 0 89
300K (without losses).
It is obvious, that fig.2.8.1 illustrates Carnot cycle, nobody
displaces cylinder with working substance from hot reservoir to cold one
and v.v. The first heat engines were steam engines. In these engines the hot
steam (working substance) was intaken into cylinder and after exhausts it
was outtaken. Another type of heat engines are internal-combustion
engines
In the common gasoline engine a mixture of air and gasoline vapor is
drawn into a cylinder through an open intake valve while the piston
descends, increasing the volume of the cylinder from a minimum of V
(when the piston is all the way up) to a maximum of RV (when it is all the
way down). The ratio R is called the compression ratio, and for present-
day automobile engines it is typically about 8. At the end of this intake
stroke the intake valve closes and the mixture is compressed to volume V
during the compression stroke. The mixture is then ignited by the spark
plug, and the heated gas expands back to volume RV, pushing on the
piston and doing work; this is the power stroke. Finally, the exhaust valve
opens and the combustion products are pushed out (during the exhaust
stroke) to prepare the cylinder for the next intake stroke
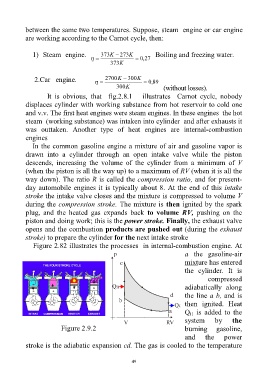
Figure 2.82 illustrates the processes in internal-combustion engine. At
a the gasoline-air
mixture has entered
the cylinder. It is
compressed
adiabatically along
the line a b, and is
then ignited. Heat
Q is added to the
H
system by the
Figure 2.9.2 burning gasoline,
and the power
stroke is the adiabatic expansion cd. The gas is cooled to the temperature
49