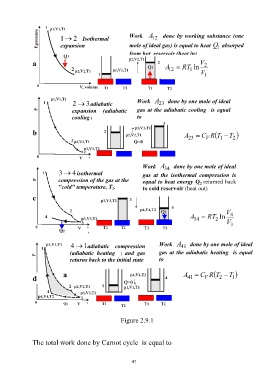
Page 47 - 4498
P. 47
1 2 Isothermal Work A done by working substance (one
12
expansion mole of ideal gas) is equal to heat Q absorped
1
from hot reservoir (heat in)
a V 2
A RT 1 ln
12
V
1
2 3adiabatic Work A done by one mole of ideal
23
expansion (adiabatic gas at the adiabatic cooling is equal
cooling ) to
b A C V R T T 2
1
23
Work A done by one mole of ideal
34
3 4isothermal gas at the isothermal compression is
compression of the gas at the equal to heat energy Q 2 returned back
"cold" temperature, T 2. to cold reservoir (heat out)
c
V
A RT 2 ln 4
34
V 3
4 1adiabatic compression Work A done by one mole of ideal
41
(adiabatic heating ) and gas gas at the adiabatic heating is equal
returns back to the initial state to
a A C R T T
d 41 V 2 1
Figure 2.9.1
The total work done by Carnot cycle is equal to
47