Page 5 - 4461
P. 5
1. Fundamental Concepts and Definitions
1.1 Thermodynamic system
When analyzing physical situations we usually focus our attention on some portion
of matter which we separate, in our minds, from the environment external to it. We
call such a portion the system. Everything outside the system which has a direct
bearing on its behavior is called the environment. Then we seek to determine the
behavior of the system by finding how it interacts with its environment.
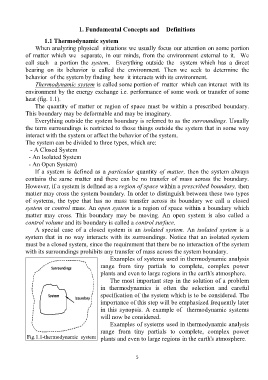
Thermodynamic system is called some portion of matter which can interact with its
environment by the energy exchange i.e. performance of some work or transfer of some
heat (fig. 1.1).
The quantity of matter or region of space must be within a prescribed boundary.
This boundary may be deformable and may be imaginary.
Everything outside the system boundary is referred to as the surroundings. Usually
the term surroundings is restricted to those things outside the system that in some way
interact with the system or affect the behavior of the system.
The system can be divided to three types, which are:
- A Closed System
- An Isolated System
- An Open System)
If a system is defined as a particular quantity of matter, then the system always
contains the same matter and there can be no transfer of mass across the boundary.
However, if a system is defined as a region of space within a prescribed boundary, then
matter may cross the system boundary. In order to distinguish between these two types
of systems, the type that has no mass transfer across its boundary we call a closed
system or control mass. An open system is a region of space within a boundary which
matter may cross. This boundary may be moving. An open system is also called a
control volume and its boundary is called a control surface.
A special case of a closed system is an isolated system. An isolated system is a
system that in no way interacts with its surroundings. Notice that an isolated system
must be a closed system, since the requirement that there be no interaction of the system
with its surroundings prohibits any transfer of mass across the system boundary.
Examples of systems used in thermodynamic analysis
range from tiny partials to complete, complex power
plants and even to large regions in the earth's atmosphere.
The most important step in the solution of a problem
in thermodynamics is often the selection and careful
specification of the system which is to be considered. The
importance of this step will be emphasized frequently later
in this synopsis. A example of thermodynamic systems
will now be considered.
Examples of systems used in thermodynamic analysis
range from tiny partials to complete, complex power
Fig.1.1-thermodynamic system plants and even to large regions in the earth's atmosphere.
5