Page 8 - 4461
P. 8
pressure increases toward the bottom of
the tank as a result of the weight of the
fluid. In many applications the variation
in pressure caused by gravity is
negligible.
Most pressure-measuring
instruments measure the difference
between the pressure of a fluid and the
pressure of the atmosphere. This
pressure difference is called gage
pressure.
The absolute pressure of the fluid is
then obtained by the relation
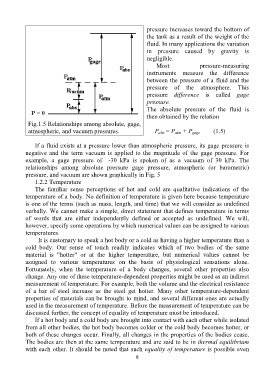
Fig.1.5 Relationships among absolute, gage,
atmospheric, and vacuum pressures. P abs = P atm + P gage (1.5)
If a fluid exists at a pressure lower than atmospheric pressure, its gage pressure is
negative and the term vacuum is applied to the magnitude of the gage pressure. For
example, a gage pressure of -30 kPa is spoken of as a vacuum of 30 kPa. The
relationships among absolute pressure gage pressure, atmospheric (or barometric)
pressure, and vacuum are shown graphically in Fig. 5
1.2.2 Temperature
The familiar sense perceptions of hot and cold are qualitative indications of the
temperature of a body. No definition of temperature is given here because temperature
is one of the terms (such as mass, length, and time) that we will consider as undefined
verbally. We cannot make a simple, direct statement that defines temperature in terms
of words that are either independently defined or accepted as undefined. We will,
however, specify some operations by which numerical values can be assigned to various
temperatures.
It is customary to speak a hot body or a cold as having a higher temperature than a
cold body. Our sense of touch readily indicates which of two bodies of the same
material is "hotter" or at the higher temperature, but numerical values cannot be
assigned to various temperatures on the basis of physiological sensations alone.
Fortunately, when the temperature of a body changes, several other properties also
change. Any one of these temperature-dependent properties might be used as an indirect
measurement of temperature. For example, both the volume and the electrical resistance
of a bar of steel increase as the steel get hotter. Many other temperature-dependent
properties of materials can be brought to mind, and several different ones are actually
used in the measurement of temperature. Before the measurement of temperature can be
discussed further, the concept of equality of temperature must be introduced.
If a hot body and a cold body are brought into contact with each other while isolated
from all other bodies, the hot body becomes colder or the cold body becomes hotter, or
both of these changes occur. Finally, all changes in the properties of the bodies cease.
The bodies are then at the same temperature and are said to be in thermal equilibrium
with each other. It should be noted that such equality of temperature is possible even
8