Page 27 - 4461
P. 27
during a complete cycle is zero, and so U U 0 .
1 2
According to the First Law of Thermodynamic:
Q U U L , as Q Q Q and U U 0 we get
1 2 1 2 1 2
Q Q L . We see that the engine receives heat energy Q 1,
1 2
but only part of it, i.e. Q Q , is converted into useful work
1 2
L. Hence, the efficiency of the heat energy is given by:
Q Q Q
2 1 2 (3.1)
Q Q
1 1
How can we make the efficiency η as great as possible? In
common engines, piston move very fast and some useful
energy is wasted in the form of heat produced by friction.
Also, they use finite differences of temperature for which the
flow of heat is not slow and so it is not reversible. Hence,
Carnot proposed a reversible cycle for an ideal heat engine
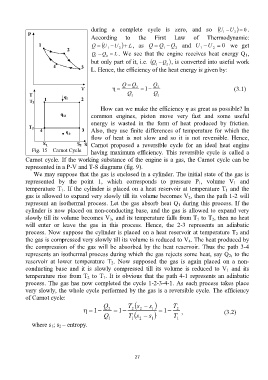
Fig. 15 – Carnot Cycle having maximum efficiency. This reversible cycle is called a
Carnot cycle. If the working substance of the engine is a gas, the Carnot cycle can be
represented in a P-V and T-S diagrams (fig. 9).
We may suppose that the gas is enclosed in a cylinder. The initial state of the gas is
represented by the point 1, which corresponds to pressure P 1, volume V 1 and
temperature T 1. If the cylinder is placed on a heat reservoir at temperature T 1 and the
gas is allowed to expand very slowly till its volume becomes V 2, then the path 1-2 will
represent an isothermal process. Let the gas absorb heat Q 1 during this process. If the
cylinder is now placed on non-conducting base, and the gas is allowed to expand very
slowly till its volume becomes V 3, and its temperature falls from T 1 to T 2, then no heat
will enter or leave the gas in this process. Hence, the 2-3 represents an adiabatic
process. Now suppose the cylinder is placed on a heat reservoir at temperature T 2 and
the gas is compressed very slowly till its volume is reduced to V 4. The heat produced by
the compression of the gas will be absorbed by the heat reservoir. Thus the path 3-4
represents an isothermal process during which the gas rejects some heat, say Q 2, to the
reservoir at lower temperature T 2. Now supposed the gas is again placed on a non-
conducting base and it is slowly compressed till its volume is reduced to V 1 and its
temperature rise from T 2 to T 1. It is obvious that the path 4-1 represents an adiabatic
process. The gas has now completed the cycle 1-2-3-4-1. As each process takes place
very slowly, the whole cycle performed by the gas is a reversible cycle. The efficiency
of Carnot cycle:
Q 2 T 2 s s 1 T 2
2
1 1 1 , (3.2)
Q T s s T
1 1 2 1 1
where s 1; s 2 – entropy.
27