Page 79 - 6641
P. 79
To 2-3 cc of aniline solution of aniline add a few
drops of chromium. As a result of the oxidation reaction of
aniline, a green color appears, which gradually changes to
blue and passes into black. The final product of oxidation of
aniline is a complex structure dye - "black aniline".
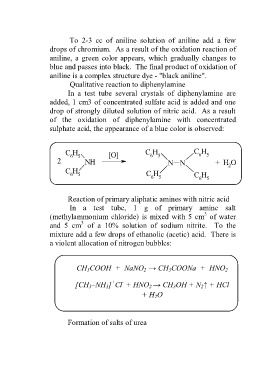
Qualitative reaction to diphenylamine
In a test tube several crystals of diphenylamine are
added, 1 cm3 of concentrated sulfate acid is added and one
drop of strongly diluted solution of nitric acid. As a result
of the oxidation of diphenylamine with concentrated
sulphate acid, the appearance of a blue color is observed:
C H C H C H
6 5 [O] 6 5 6 5
2 NH N N + H O
2
C H
6 5 C H C H
6 5
6 5
Reaction of primary aliphatic amines with nitric acid
In a test tube, 1 g of primary amine salt
3
(methylammonium chloride) is mixed with 5 cm of water
3
and 5 cm of a 10% solution of sodium nitrite. To the
mixture add a few drops of ethanolic (acetic) acid. There is
a violent allocation of nitrogen bubbles:
CH 3CH 3COOH + NaNO 2 → CH 3COONa + HNO 2
CH 3COOH + NaNO 2 → CH 3COONa + HNO 2
-
+
[CH 3–NH 3] Cl + HNO 2 → CH 3OH + N 2↑ + HCl
2O
+ H
+
-
[CH 3–NH 3] Cl + HNO 2 → CH 3OH + N 2↑ + HCl + H 2O
Formation of salts of urea