Page 20 - 4911
P. 20
Appendix B
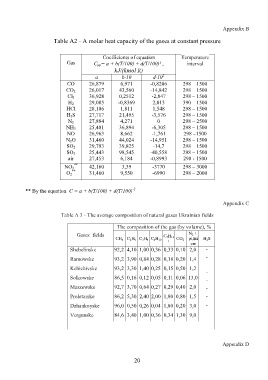
Table A2 - A molar heat capacity of the gases at constant pressure
Coefficients of equation Temperature
Gas
C μp= a + b(T/100) + d(T/100)² , interval
kJ/(kmol К) 2
a b·10 d·10
CO 26,879 6,971 -0,8206 298 – 1500
CO 2 26,017 43,560 -14,842 298 – 1500
Cl 2 36,928 0,2512 -2,847 298 – 1500
H 2 29,085 -0,8369 2,013 390 – 1500
HCl 28,186 1,811 1,548 298 – 1500
H 2S 27,717 21,495 -3,576 298 – 1500
N 2 27,884 4,271 0 298 – 2500
NH 3 25,481 36,894 -6,305 298 – 1500
NO 26,963 8,662 -1,761 298 –1500
N 2O 31,460 44,024 -14,951 298 – 1500
SO 2 29,793 39,825 -14,7 298 – 1500
SO 3 25,443 98,545 -40,558 298 – 1500
air 27,453 6,184 -0,8993 298 - 1500
**
NO 2 42,160 3,39 -3770 298 – 3000
O 2 ** 31,460 9,550 -6990 298 – 2000
** By the equation C = a + b(T/100) + d(T/100) -2
Appendix C
Table A 3 - The average composition of natural gases Ukrainian fields
The composition of the gas (by volume), %
Gases fields С 5Н 12 N 2 +
СН 4 С 2Н 6 С 3Н 8 С 4Н 10 СО 2 рідкі Н 2S
+
сні
Shebelinske 92,2 4,10 1,00 0,36 0,33 0,10 2,0 -
-
Ramowske 93,2 3,90 0,84 0,28 0,18 0,20 1,4
Kehichivske 93,2 3,30 1,40 0,25 0,15 0,50 1,2
-
Solkowske 86,5 0,16 0,12 0,05 0,11 0,06 13,0
-
Maszewske 92,7 3,70 0,64 0,27 0,29 0,40 2,0 -
Proletarske 86,2 5,30 2,40 2,00 1,80 0,80 1,5 -
Dzhankoyske 96,0 0,50 0,26 0,04 1,80 0,20 3,0 -
Vergunske 84,6 3,40 1,00 0,36 0,34 1,30 9,0
Appendix D
20