Page 19 - 4911
P. 19
Appendix A
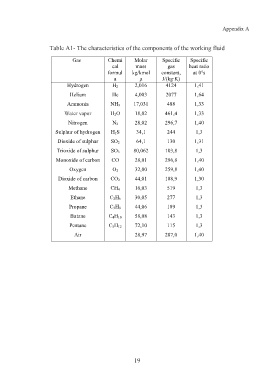
Table A1- The characteristics of the components of the working fluid
Gas Chemi Molar Specific Specific
cal mass gas heat ratio
formul kg/kmol constant, at 0°s
a μ J/(kg·K)
Hydrogen H 2 2,016 4124 1,41
Helium He 4,003 2077 1,64
Ammonia NH 3 17,031 488 1,33
Water vapor H 2O 18,02 461,4 1,33
Nitrogen N 2 28,02 296,7 1,40
Sulphur of hydrogen H 2S 34,1 244 1,3
Dioxide of sulphur SO 2 64,1 130 1,31
Trioxide of sulphur SO 3 80,062 103,8 1,3
Monoxide of carbon CO 28,01 296,8 1,40
Oxygen O 2 32,00 259,8 1,40
Dioxide of carbon CO 2 44,01 188,9 1,30
Methane СН 4 16,03 519 1,3
Ethane С 2Н 6 30,05 277 1,3
Propane С 3Н 8 44,06 189 1,3
Butane С 4Н 10 58,08 143 1,3
Pentane С 5Н 12 72,10 115 1,3
Air — 28,97 287,0 1,40
19