Page 13 - 4638
P. 13
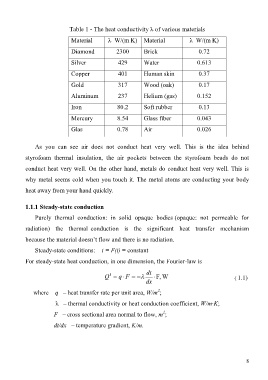
Table 1 - The heat conductivity λ of various materials
Material λ W/(m K) Material λ W/(m K)
Diamond 2300 Brick 0.72
Silver 429 Water 0.613
Copper 401 Human skin 0.37
Gold 317 Wood (oak) 0.17
Aluminum 237 Helium (gas) 0.152
Iron 80.2 Soft rubber 0.13
Mercury 8.54 Glass fiber 0.043
Glas 0.78 Air 0.026
As you can see air does not conduct heat very well. This is the idea behind
styrofoam thermal insulation, the air pockets between the styrofoam beads do not
conduct heat very well. On the other hand, metals do conduct heat very well. This is
why metal seems cold when you touch it. The metal atoms are conducting your body
heat away from your hand quickly.
1.1.1 Steady-state conduction
Purely thermal conduction: in solid opaque bodies (opaque: not permeable for
radiation) the thermal conduction is the significant heat transfer mechanism
because the material doesn’t flow and there is no radiation.
Steady-state conditions: t = F(t) = constant
For steady-state heat conduction, in one dimension, the Fourier-law is
dt
I
Q q F F, W ( 1.1)
dx
2
where q – heat transfer rate per unit area, W/m ;
λ – thermal conductivity or heat conduction coefficient, W/m·K;
2
F – cross sectional area normal to flow, m ;
dt/dx – temperature gradient, K/m.
8