Page 11 - 4638
P. 11
Figure 2 – Conduction heat transfer modes
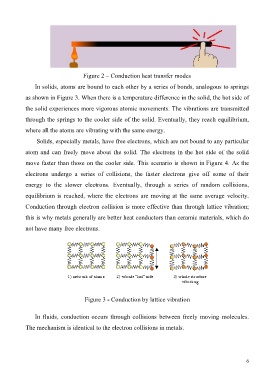
In solids, atoms are bound to each other by a series of bonds, analogous to springs
as shown in Figure 3. When there is a temperature difference in the solid, the hot side of
the solid experiences more vigorous atomic movements. The vibrations are transmitted
through the springs to the cooler side of the solid. Eventually, they reach equilibrium,
where all the atoms are vibrating with the same energy.
Solids, especially metals, have free electrons, which are not bound to any particular
atom and can freely move about the solid. The electrons in the hot side of the solid
move faster than those on the cooler side. This scenario is shown in Figure 4. As the
electrons undergo a series of collisions, the faster electrons give off some of their
energy to the slower electrons. Eventually, through a series of random collisions,
equilibrium is reached, where the electrons are moving at the same average velocity.
Conduction through electron collision is more effective than through lattice vibration;
this is why metals generally are better heat conductors than ceramic materials, which do
not have many free electrons.
Figure 3 - Conduction by lattice vibration
In fluids, conduction occurs through collisions between freely moving molecules.
The mechanism is identical to the electron collisions in metals.
6