Page 56 - 6273
P. 56
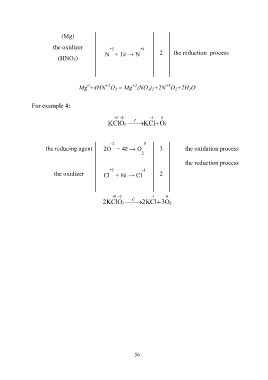
(Mg)
the oxidizer +5 +4
N + 1ē → N 2 the reduction process
(HNO 3)
+5
+2
0
+4
Mg +4HN O 3 = Mg (NO 3) 2+2N O 2+2H 2O
For example 4:
5 2 o -1 0
K Cl O 3 t K 2 O Cl
-2 0
the reducing agent 2O − 4ē → O 3 the oxidation process
2
the reduction process
+5 -1
the oxidizer Сl + 6ē → Cl 2
5 2 o -1 0
2K Cl O 3 t 2K Cl 3 2 O
56