Page 83 - 6273
P. 83
3-
-
2-
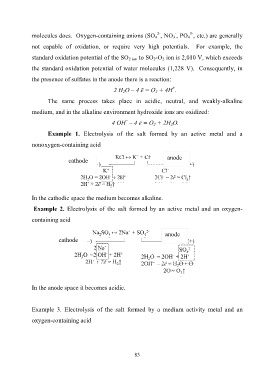
molecules does. Oxygen-containing anions (SO 4 , NO 3 , PO 4 , etc.) are generally
not capable of oxidation, or require very high potentials. For example, the
standard oxidation potential of the SO 2 ion to SO 2-O 2 ion is 2,010 V, which exceeds
the standard oxidation potential of water molecules (1,228 V). Consequently, in
the presence of sulfates in the anode there is a reaction:
+
2 Н 2О – 4 ē = О 2 + 4Н .
The same process takes place in acidic, neutral, and weakly-alkaline
medium, and in the alkaline environment hydroxide ions are oxidized:
–
4 ОН – 4 ē = О 2 + 2Н 2О.
Example 1. Electrolysis of the salt formed by an active metal and a
nonoxygen-containing acid
anode
cathode
In the cathodic space the medium becomes alkaline.
Example 2. Electrolysis of the salt formed by an active metal and an oxygen-
containing acid
anode
cathode
In the anode space it becomes acidic.
Example 3. Electrolysis of the salt formed by a medium activity metal and an
oxygen-containing acid
83