Page 15 - 4461
P. 15
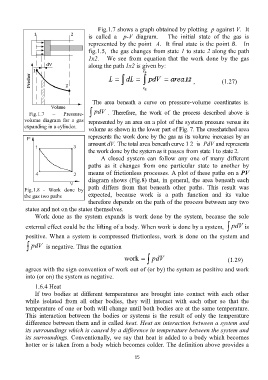
Fig.1.7 shows a graph obtained by plotting p against V. It
is called a p-V diagram. The initial state of the gas is
represented by the point A. It final state is the point B. In
fig.1.5, the gas changes from state 1 to state 2 along the path
1x2. We see from equation that the work done by the gas
along the path 1x2 is given by:
, (1.27)
The area beneath a curve on pressure-volume coordinates is.
Fig.1.7 – Pressure- pdV . Therefore, the work of the process described above is
volume diagram for a gas represented by an area on a plot of the system pressure versus its
expanding in a cylinder.
volume as shown in the lower part of Fig. 7. The crosshatched area
represents the work done by the gas as its volume increases by an
amount dV. The total area beneath curve 1 2 is PdV and represents
the work done by the system as it passes from state 1 to state 2.
A closed system can follow any one of many different
paths as it changes from one particular state to another by
means of frictionless processes. A plot of these paths on a PV
diagram shows (Fig.8) that, in general, the area beneath each
Fig.1.8 - Work done by path differs from that beneath other paths. This result was
the gas two paths expected, because work is a path function and its value
therefore depends on the path of the process between any two
states and not on the states themselves.
Work done as the system expands is work done by the system, because the sole
external effect could be the lifting of a body. When work is done by a system, pdV is
positive. When a system is compressed frictionless, work is done on the system and
pdV is negative. Thus the equation
work pdV (1.29)
agrees with the sign convention of work out of (or by) the system as positive and work
into (or on) the system as negative.
1.6.4 Heat
If two bodies at different temperatures are brought into contact with each other
while isolated from all other bodies, they will interact with each other so that the
temperature of one or both will change until both bodies are at the same temperature.
This interaction between the bodies or systems is the result of only the temperature
difference between them and is called heat. Heat an interaction between a system and
its surroundings which is caused by a difference in temperature between the system and
its surroundings. Conventionally, we say that heat is added to a body which becomes
hotter or is taken from a body which becomes colder. The definition above provides a
15